✅ Edited and Verified by the Epcland Engineering Team
Types of Corrosion in Piping: The Complete Engineering Guide
Imagine a Carbon Steel process line operating at 90°C. The aluminum cladding looks pristine, but beneath the surface, moisture has trapped itself against the hot pipe. This is Corrosion Under Insulation (CUI)—a “hidden killer” that forces unplanned shutdowns. In piping engineering, corrosion isn’t just surface rust; it is the leading cause of asset failure. This guide breaks down the critical types of corrosion you must identify and mitigate in EPC projects.
🧪 Pre-Read Quiz: Test Your Corrosion Knowledge
Table of Contents
Complete Course on
Piping Engineering
Check Now
Key Features
- 125+ Hours Content
- 500+ Recorded Lectures
- 20+ Years Exp.
- Lifetime Access
Coverage
- Codes & Standards
- Layouts & Design
- Material Eng.
- Stress Analysis
🔍 Visual Diagnostic: What does the damage look like?
Identify the corrosion type based on visual evidence and location.
| Visual Symptom | Likely Corrosion Type | Common Location |
|---|---|---|
| Uniform thinning / Rust everywhere | General/Uniform | Uncoated carbon steel exposed to weather or process. |
| Deep, narrow holes / “Pinholes” | Pitting | Stagnant lines, drains, or Stainless Steel with chlorides. |
| Severe metal loss at flange/joint | Galvanic | Connection between CS (Pipe) and SS (Valve/Trim). |
| Grooves, waves, or “horseshoe” marks | Erosion | Downstream of pumps, control valves, or elbows (high velocity). |
| Fine branching cracks (Spiderweb) | SCC (Stress Cracking) | Hot insulated lines, caustic service, or high-stress zones. |
| Corrosion found *under* insulation | CUI | Lines operating between -4°C and 175°C with damaged cladding. |
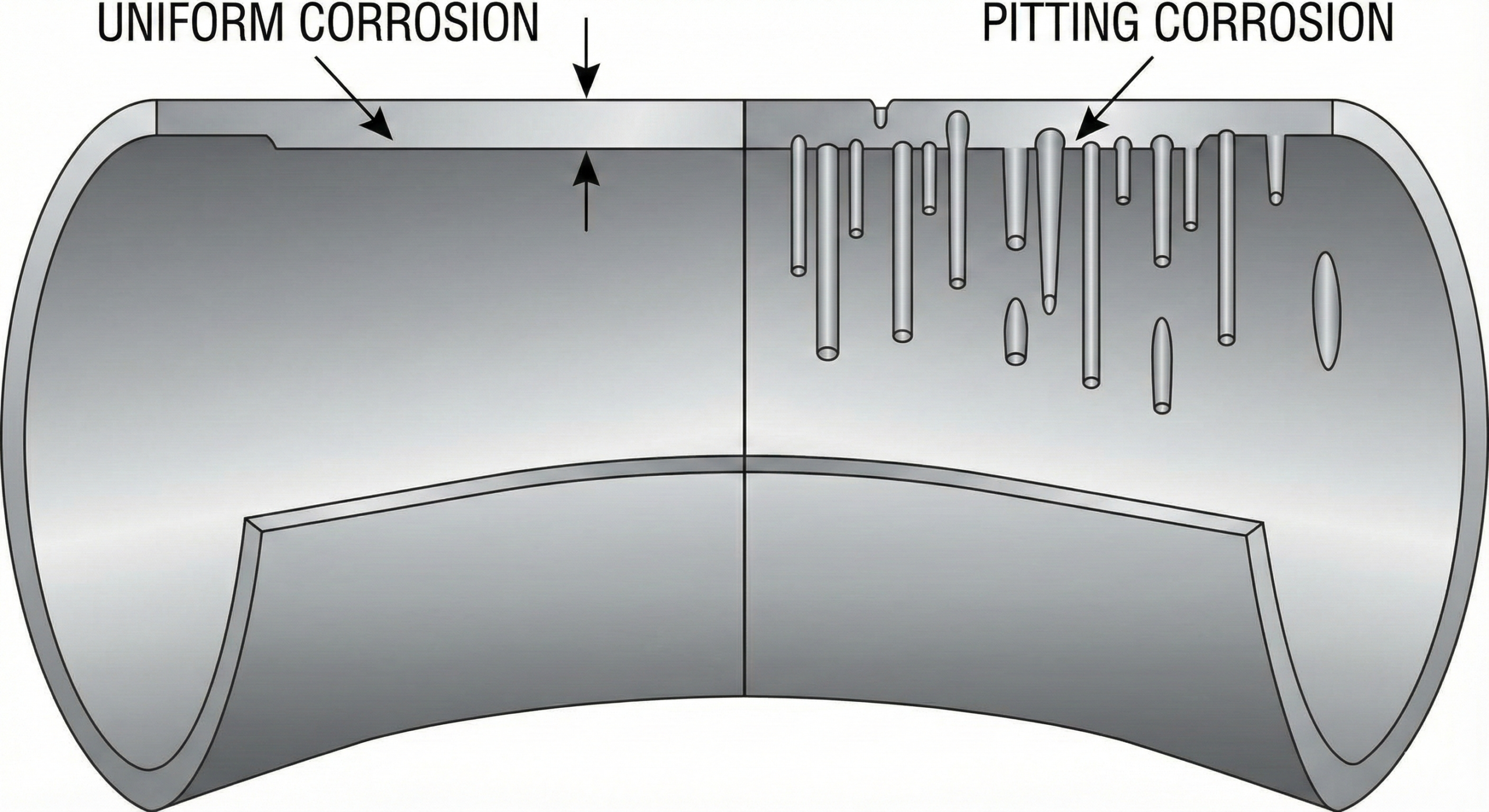
1. General (Uniform) Corrosion
General corrosion is the most common and, ironically, the “safest” form of corrosion because it is predictable. It attacks the entire surface area of the pipe uniformly, leading to a gradual reduction in wall thickness. This is typically caused by chemical or electrochemical reactions between the pipe material (usually Carbon Steel) and the environment (atmosphere or fluid).
Engineering Defense: This is primarily managed by adding a Corrosion Allowance (CA)—extra wall thickness added during the design phase (typically 1.5mm to 6mm) to last the design life of the plant.
2. Galvanic Corrosion (Dissimilar Metals)
Referencing our context, this occurs when two different metals (e.g., Stainless Steel and Carbon Steel) are electrically connected in the presence of an electrolyte (like salt water). The metal with the lower electrical potential (the anode/Carbon Steel) corrodes rapidly to protect the metal with the higher potential (the cathode/Stainless Steel).
The Rule of Thumb: Never connect CS to SS directly in wet environments. Use Insulating Flange Kits or dielectric unions to break the electrical path.
3. Pitting & Crevice Corrosion
Unlike uniform corrosion, pitting is a localized attack that creates deep holes in the metal. It is particularly dangerous because a pipe can fail (leak) while the majority of the surface looks shiny and new.
- Pitting: Often caused by chloride ions breaking down the passive film on Stainless Steel.
- Crevice Corrosion: Occurs in stagnant areas like gaps between gaskets and flanges, or under bolt heads, where the solution becomes stagnant and acidic.
“The most expensive corrosion is the one you didn’t see coming. A uniform loss of 1mm over 10 years is manageable; a 1mm pit penetrating the wall in 6 months is catastrophic.”
4. The Hidden Killer: Corrosion Under Insulation (CUI)
CUI is arguably the biggest maintenance headache in the petrochemical industry. It occurs when water breaches the external weather jacketing (cladding) and soaks the insulation material. The wet insulation holds moisture against the hot pipe surface, creating a perfect corrosion cell.
Critical Zone: Carbon Steel operating between 60°C and 175°C is most at risk because water continuously evaporates and condenses, concentrating corrosive salts.
5. Stress Corrosion Cracking (SCC)
SCC is a brittle failure mode that occurs due to the combined effect of tensile stress and a corrosive environment. The classic example in piping is Chloride Stress Corrosion Cracking (Cl-SCC) in 300-series Stainless Steels (like SS304/316) operating above 60°C. The metal doesn’t rust away; it cracks instantly and catastrophically.
7. Calculation: Remaining Life Assessment
As an engineer, you must quantify corrosion to make “Replace vs. Repair” decisions. Here is how we calculate the Remaining Life of a piping asset based on ultrasonic thickness (UT) measurements.
🧮 Solved Example: Pipe Remaining Life
Scenario: A Carbon Steel pipe (NPS 10, Sch 40) had a nominal thickness of 9.27 mm in 2015. In 2025, inspection shows an actual thickness of 7.50 mm. The minimum required structural thickness (Tmin) is 5.0 mm.
Step 1: Calculate Corrosion Rate (CR)
CR = (9.27 mm – 7.50 mm) / 10 Years
CR = 1.77 / 10 = 0.177 mm/year
Step 2: Calculate Remaining Life (RL)
RL = (7.50 mm – 5.0 mm) / 0.177 mm/year
RL = 2.5 / 0.177 ≈ 14.1 Years
Result: The pipe is safe for continued operation for approximately 14 more years, assuming the corrosion rate remains constant.
EPCLand YouTube Channel
2,500+ Videos • Daily Updates
⚡ The Galvanic Series (Simplified)
When connecting two metals, the one higher on this list (Anodic) will corrode to protect the one lower (Cathodic).
Anodic (Active)
Sacrificial / Corrodes
- Magnesium
- Zinc
- Aluminum
- Carbon Steel
- Cast Iron
Transition
- Brass / Bronze
- Copper
- Nickel Alloys
Cathodic (Noble)
Protected / Survives
- Stainless Steel (304/316)
- Titanium
- Gold / Platinum
🛡️ Corrosion Prevention Strategy Matrix
| Corrosion Type | Design Solution | Material / Coating Fix |
|---|---|---|
| General / Uniform | Add Corrosion Allowance (CA) | Paint/Coating or Cathodic Protection (CP) |
| Galvanic | Install Insulation Kits (Flanges) | Match materials or coat the Cathode |
| Pitting | Eliminate stagnant zones (Dead legs) | Use High-Molybdenum alloys (e.g., SS316, Duplex) |
| CUI | Design insulation to drain water | TSA (Thermally Sprayed Aluminum) coating |
| Erosion | Reduce fluid velocity / Increase radius | Harder materials (Stellite overlay) |
| Crevice | Use welded joints instead of flanges | Fill gaps with sealant |
Frequently Asked Questions (FAQ)
What is the most effective piping material for seawater service?
While Carbon Steel corrodes rapidly in seawater, materials like Cupro-Nickel (90/10), Duplex Stainless Steel (2205), and Super Duplex are standard choices due to their high resistance to chloride pitting and crevice corrosion. Titanium is used for extreme conditions but is costly.
How can we detect CUI without removing the insulation?
Stripping insulation is expensive. Modern Non-Destructive Examination (NDE) methods include Pulsed Eddy Current (PEC), Real-time Radiography (RTR), and Guided Wave Ultrasonics. These allow engineers to scan for wall loss through the insulation layer.
What is the difference between Mill Tolerance and Corrosion Allowance?
Corrosion Allowance is a design buffer added to account for material loss over time (e.g., 3mm). Mill Tolerance is a manufacturing variation allowed by ASTM standards (e.g., 12.5% under-thickness for seamless pipe). Both must be subtracted when calculating the minimum required thickness.
Does painting/coating eliminating the need for Corrosion Allowance?
No. Coatings are considered a “passive” barrier that can fail or get damaged during installation. Most design codes (like ASME B31.3) still require a calculated corrosion allowance based on the fluid corrosivity and design life, regardless of painting.
Conclusion
Corrosion is inevitable, but failure is optional. As we saw with the “Hidden Killer” CUI scenario, relying solely on visual inspection is a recipe for disaster. Whether you are dealing with the rapid attack of galvanic corrosion or the stealthy propagation of Stress Corrosion Cracking, the solution lies in the Design Phase.
By selecting the right materials, applying correct isolation kits, and calculating accurate corrosion allowances, you ensure the integrity of the piping system. Remember: In piping engineering, a small investment in corrosion control today saves millions in shutdown costs tomorrow.
🚀 Master Piping Engineering?
Don’t just read about it. Get the full industry-ready training.
View Complete CourseAbout the Author: This guide was developed by the Epcland Engineering Team, a collective of Senior Piping Engineers and Stress Analysts dedicated to practical, field-verified engineering education.







