What Is White Rust? Its Formation, White Rust Prevention, and Professional Treatment

You’ve just received a multi-million dollar shipment of galvanized structural steel, but upon opening the bundles, you find the surface covered in a waxy, white chalky powder. Is it a superficial cosmetic issue, or is the protective zinc coating fundamentally compromised? In the world of EPC projects, this “white bloom” is more than an eyesore; it is a race against time. Without immediate White Rust Prevention strategies, your materials can face rapid depletion of their sacrificial protection, leading to rejected inspections and massive project delays.
Key Project Takeaways
- Chemical Shift: White rust is Zinc Hydroxide, formed when zinc reacts with pure water in an oxygen-depleted environment, preventing the formation of protective Zinc Carbonate.
- Critical Standard: Compliance with ASTM A123 and proper passivation is the first line of defense in White Rust Prevention.
- Recovery Protocol: Light staining can be remediated with nylon brushing, but heavy “black” deposits often require complete stripping and re-galvanizing.
What is White Rust?
White rust, or wet storage stain, is a corrosion product (Zinc Hydroxide) that forms on galvanized steel when the surface is exposed to moisture in stagnant, poorly ventilated conditions. Effective White Rust Prevention relies on maintaining air circulation and using chemical passivates to allow a stable Zinc Carbonate patina to develop.
Founder’s Insight
“In my 20 years of site inspections, I have seen more galvanized steel ruined by improper storage than by actual service-life wear. White Rust Prevention isn’t just about chemicals; it’s about the logistics of how you stack and vent your materials on-site.”
— Atul Singla
Table of Contents
- 1. What is White Rust (Zinc Hydroxide)?
- 2. Chemical Mechanisms: What Causes White Rust?
- 3. Is White Rust Bad for Galvanized Steel?
- 4. Engineering Standards for White Rust Prevention
- 5. Strategic White Rust Prevention in Storage
- 6. Repairing and Advanced White Rust Treatment
- 7. Conclusion: Proactive Prevention Costs
Complete Course on
Piping Engineering
Check Now
Key Features
- 125+ Hours Content
- 500+ Recorded Lectures
- 20+ Years Exp.
- Lifetime Access
Coverage
- Codes & Standards
- Layouts & Design
- Material Eng.
- Stress Analysis
Engineering Competency: White Rust Prevention
Test your knowledge on Zinc Hydroxide and ASTM standards.
What is the primary chemical composition of “White Rust”?
What is White Rust (Zinc Hydroxide)?
In the context of hot-dip galvanizing, white rust—scientifically identified as Zinc Hydroxide—is a corrosive byproduct that occurs when the highly reactive zinc surface is prevented from forming its natural protective patina. Under normal atmospheric conditions, a freshly galvanized surface reacts with oxygen to form zinc oxide, which then reacts with moisture and carbon dioxide to form a thin, hard, and stable layer of Zinc Carbonate (ZnCO3). This patina is what gives galvanized steel its legendary multi-decade durability.
However, when White Rust Prevention measures fail, and the steel is exposed to pure water (rain, condensation, or dew) in a stagnant, oxygen-depleted environment, the zinc cannot find the carbon dioxide necessary to complete its chemical transition. Instead, it reacts continuously with water to form a porous, waxy, and voluminous accumulation of zinc hydroxide. Unlike the protective patina, this white substance is non-adherent and offers no protection against further corrosion.
Chemical Mechanisms: What Causes White Rust?
The fundamental cause of white rust is the presence of “Pure Water” (water without dissolved minerals or salts) trapped against the zinc surface. This often occurs during “Wet Storage Stain” conditions. For successful White Rust Prevention, engineers must understand the specific environmental triggers that accelerate this degradation:
- Tightly Nested Bundling: When flat sheets or pipes are stacked directly against one another, capillary action draws moisture into the gaps, where it remains trapped without airflow.
- Rapid Temperature Fluctuations: Moving cold galvanized steel into a warm warehouse creates “sweating” or condensation, which is the purest form of water and highly corrosive to unpassivated zinc.
- Inadequate Passivation: Newly galvanized steel is most vulnerable for the first 48 hours to 6 weeks. If the galvanizer skips the chromate or phosphate passivation bath, the “virgin” zinc has no temporary chemical barrier.
- Airflow Stagnation: Without CO2 replenishment, the protective zinc carbonate layer cannot form, leaving the zinc in a state of perpetual hydroxide formation.
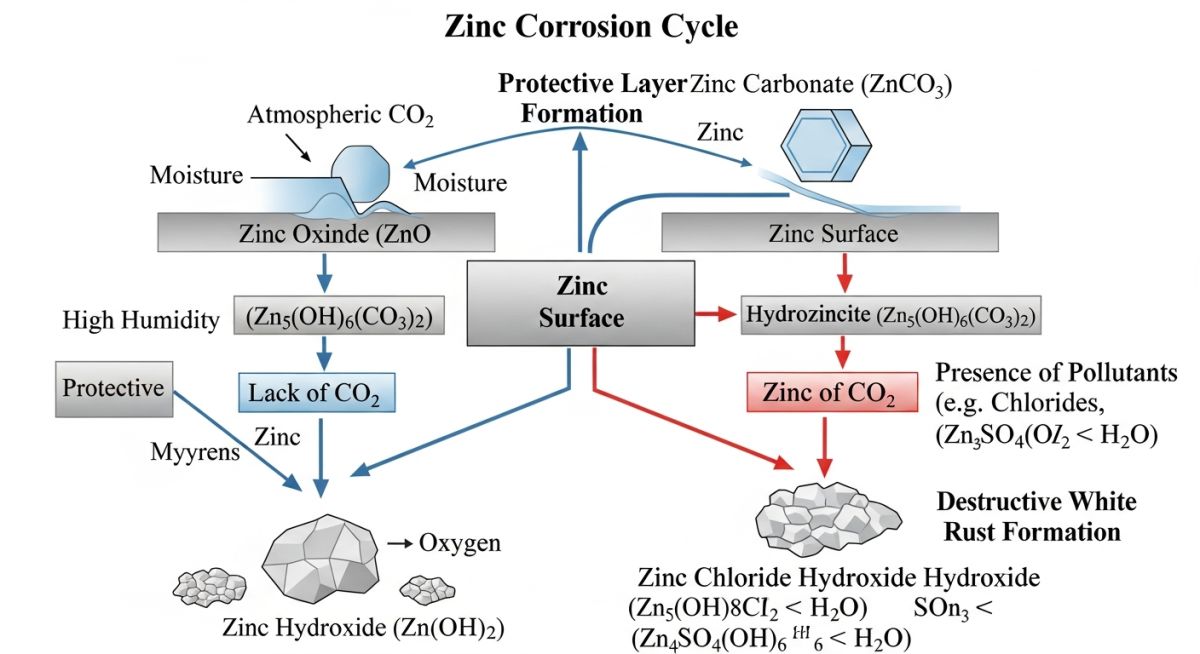
Figure 1: The bifurcation of Zinc oxidation—Path A leads to protective Zinc Carbonate, while Path B (Low CO2 + High Moisture) leads to destructive Zinc Hydroxide.
Structural Integrity: Is White Rust Bad for Galvanized Steel?
The impact of white rust on structural integrity depends entirely on the Depth of Attack. Engineering inspectors typically categorize the severity into three distinct levels to determine if White Rust Prevention was successful or if remediation is required:
- Light White Rust: Characterized by a light film of white powder. It is purely cosmetic and rarely affects the coating thickness. If the moisture source is removed, it may eventually convert to a stable patina.
- Medium White Rust: Notable darkening of the zinc surface with heavy, waxy deposits. At this stage, the zinc coating is being actively consumed. It requires mechanical cleaning and a Dry Film Thickness (DFT) check to ensure compliance with ASTM A123.
- Severe White Rust (Black Stain): The presence of black or dark gray spots indicates that the zinc coating has been significantly depleted, potentially exposing the underlying steel-zinc alloy layers. If the DFT falls below the minimum required for the specific steel thickness, the piece must be rejected and re-galvanized.
Engineering Standards for White Rust Prevention
In the engineering and construction sector, White Rust Prevention is not merely a recommendation but a requirement governed by international standards. The most critical benchmark is ASTM A123 (Standard Specification for Zinc/Hot-Dip Galvanized Coatings on Iron and Steel Products). While ASTM A123 states that “wet storage stain” is not typically a cause for rejection unless the coating thickness is compromised, it places the onus on the handler to maintain environmental controls.
Other relevant standards include ASTM A153 for hardware and ASTM A780, which provides the protocol for repairing damaged galvanized coatings. For White Rust Prevention to be contractually compliant, the following engineering criteria must be met:
| Standard | Application | White Rust Implication |
|---|---|---|
| ASTM A123 | Structural Steel Sections | Requires minimum DFT; stain allowed if DFT is within spec. |
| ASTM A153 | Fasteners and Hardware | Threaded parts must be clean to ensure proper fit-up. |
| ASTM A780 | Repair of HDG Coatings | Defines the use of zinc-rich paints for severe rust repair. |
| ISO 1461 | Global Galvanizing Code | Specifies that storage conditions are the user’s responsibility. |
Strategic White Rust Prevention in Storage and Transport
The most effective White Rust Prevention occurs long before the steel arrives on site. Logistics teams must implement a rigorous storage protocol to ensure air circulation and moisture runoff.
- Incined Stacking: Steel should be stored at a minimum 10° to 15° angle to allow water to drain immediately rather than pooling on flat surfaces.
- The “Lumber Crayon” Rule: Use spacers (dunnage) made of non-absorbent materials or plastic-wrapped wood between nested items to break capillary tension.
- Avoid Tarping: Never wrap galvanized steel in plastic or tarps, as these trap humidity and create a “greenhouse effect” that accelerates White Rust Prevention failure.
- Indoor Maturation: If possible, store newly galvanized steel indoors for the first 48 hours to allow the initial oxide layer to stabilize before outdoor exposure.
Repairing White Rust and Advanced White Rust Treatment
If White Rust Prevention fails, the remediation path is determined by the severity of the Zinc Hydroxide buildup. In 2026, the industry-standard approach for White Rust Treatment involves:
Stage 1: Mechanical Cleaning
Use a stiff-bristle nylon brush (avoid wire brushes, which can contaminate the zinc) to scrub the white deposits. In many cases, removing the hydroxide allows the carbonation process to restart.
Stage 2: Chemical Passivation
Apply a mild acidic solution (like white vinegar or a proprietary citric acid cleaner) to neutralize the hydroxide, followed by a thorough fresh-water rinse and rapid drying.
Stage 3: Zinc-Rich Restoration
For severe cases where the DFT is compromised, the area must be cleaned to SP-10 (Near-White Blast) and coated with a 95% zinc-rich primer in accordance with ASTM A780.
Galvanized Coating Health & White Rust Loss Calculator
Estimate the remaining service life after White Rust occurrence based on ASTM A123 standards for 2026.
Estimated Remaining Thickness
Input your data to see the White Rust Prevention remediation strategy.
EPCLand YouTube Channel
2,500+ Videos • Daily Updates
Don’t miss this video related to corrosion

Summary: Welcome to our comprehensive 30-day course on ASME B31.3 – the code that governs process piping! 🛢️ In this single video, ……
White Rust Prevention Failure Case Study

Project Context: Coastal HVAC Infrastructure
In a 2026 infrastructure project located in a high-humidity coastal region, 45 tons of hot-dip galvanized structural support beams were delivered and staged for installation. Due to a three-week scheduling delay, the beams remained in their shipping bundles, tightly nested and wrapped in heavy-duty polyethylene (plastic) sheeting to “protect” them from the salt air.
The Failure Mechanism
The plastic wrapping created a Micro-Greenhouse Effect. Diurnal temperature swings caused heavy condensation to form on the interior of the plastic. Because the beams were stacked flat (0° incline) and tightly nested, the water was drawn between the beams via capillary action. With no airflow and no CO2 availability, the zinc surface entered a rapid Zinc Hydroxide (White Rust) cycle.
Inspection Results:
- Visual: 65% of the surface area covered in waxy white “bloom.”
- Coating Loss: Pre-shipment DFT was 105μm; post-failure DFT dropped to 62μm in localized areas.
- Compliance: The steel fell below the ASTM A123 minimum requirement of 85μm for this material thickness.
The Remediation & Lesson Learned
The total cost of remediation exceeded $22,000, including mechanical cleaning, acid-wash neutralization, and localized repair with 95% zinc-rich cold galvanizing compounds. This failure highlights that White Rust Prevention is a logistical discipline: if the beams had been stored at an incline with 50mm spacers (dunnage) and no plastic wrapping, the stable zinc carbonate patina would have formed naturally, saving the project 14 days of downtime.
Expert Insights: Lessons from 20 Years in the Field
Reflecting on two decades of corrosion engineering, these are the critical non-negotiables for White Rust Prevention in 2026:
- The First 48 Hours are Vital: The vulnerability of zinc is at its peak immediately after the centrifuge or quench. Ensuring steel stays dry for the first two days is more effective than any chemical treatment applied later.
- Avoid “Air-Tight” Packaging: I have seen more damage from “protection” (shrink wrap/tarps) than from leaving steel exposed to the rain. If you must cover it, use a breathable canvas or tent the tarp to allow a chimney effect for airflow.
- The Fingerprint Factor: Oils from hands can disrupt the formation of the zinc carbonate patina. In high-spec architectural galvanizing, workers should wear clean gloves during the initial stacking to ensure uniform White Rust Prevention.
- Specify Passivation: Never assume a galvanizer will passivate your steel. Explicitly request a chromate or high-performance chrome-free passivation in your purchase order to provide that critical temporary shield.
Frequently Asked Questions
Can white rust turn back into a normal protective coating? ▼
Does white rust mean the galvanizing was done poorly? ▼
Is it safe to paint over white rust? ▼
What is the “Human Hook” indicator for severe failure? ▼
How do I prove the steel is still usable after treatment? ▼
Which chemical passivates are recommended in 2026? ▼
📚 Recommended Resources: corrosion
Read these Guides
🎓 Advanced Training
🎥 Watch Tutorials







