Master Wing Nut Types and Applications. Explore ASME standards, material selection, and 2026 industrial use cases for tool-free fasteners.


Master Wing Nut Types and Applications. Explore ASME standards, material selection, and 2026 industrial use cases for tool-free fasteners.
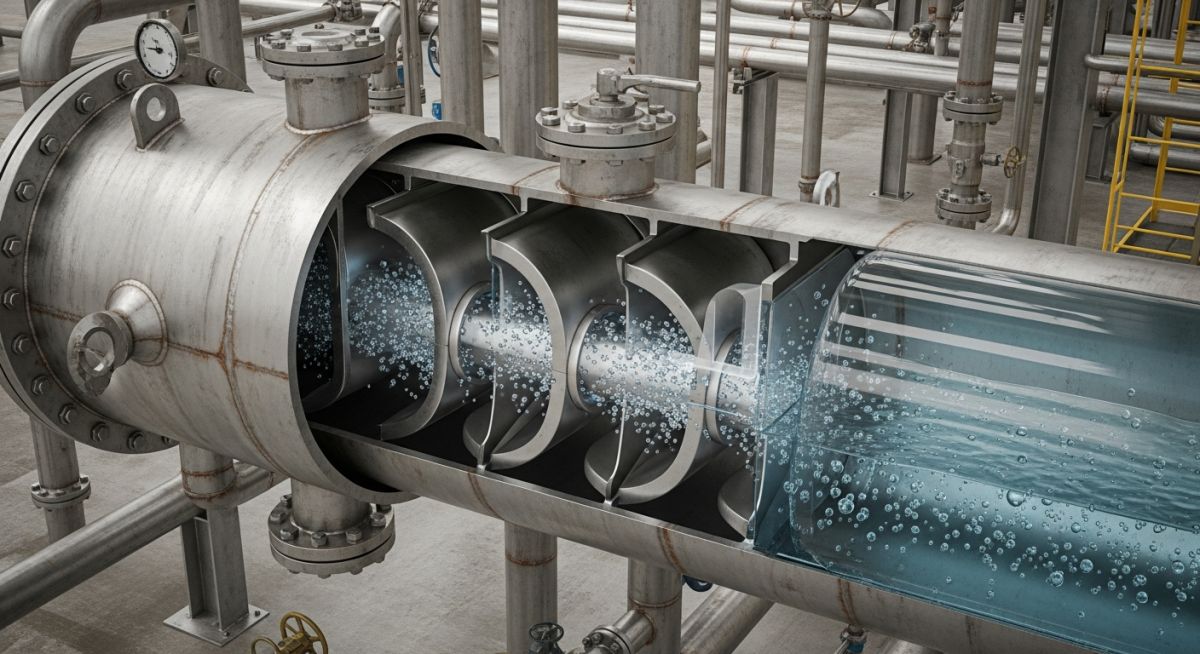
Master the fundamentals of coalescers. Learn how these critical engineering devices separate emulsions, protect equipment, and ensure fluid purity in 2026.

Master underground piping insulation. Explore ASME/API standards, material selection, and direct burial techniques for 2026 engineering projects.
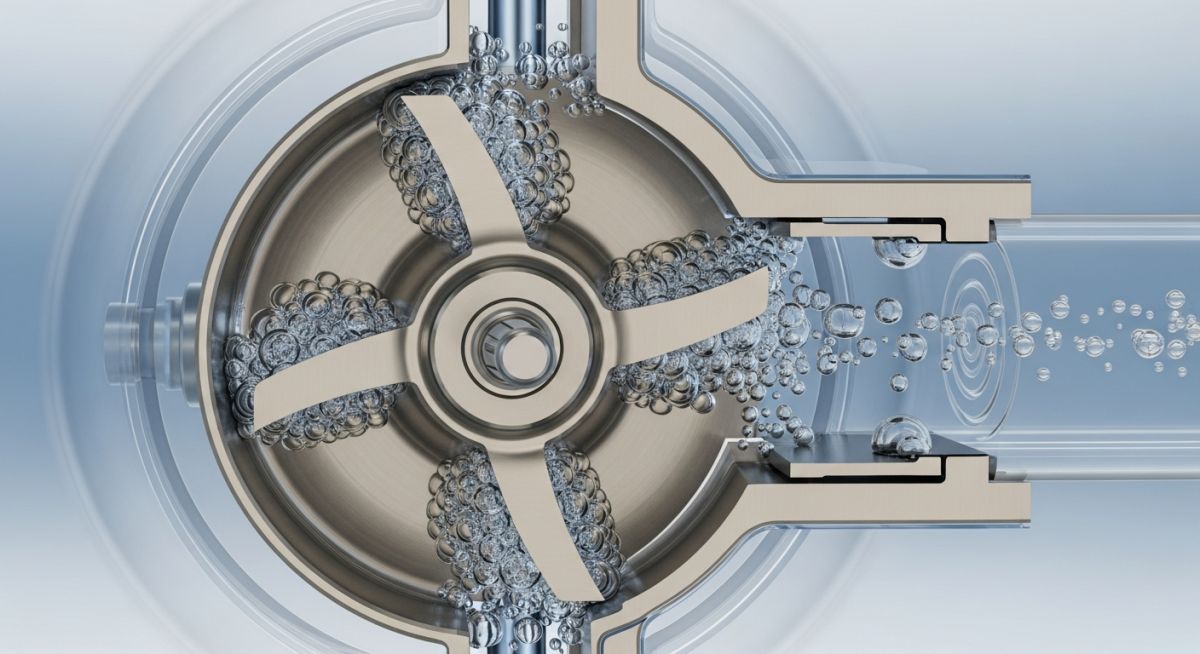
Master centrifugal pump cavitation prevention. Learn NPSH calculations, vibration analysis, and engineering fixes to protect your assets in 2026.

Confused about Washers vs Gaskets? Learn the engineering differences in load distribution, sealing mechanics, and material selection according to ASME standards.

Master the thermodynamics, industrial properties and applications of condensate. Learn how to optimize heat recovery and ASME standards for 2026.

Master the engineering of Socket Weld Pipe Fittings. Learn ASME B16.11 standards, installation gaps, and why these small-bore components are critical.

Master the essentials of TEMA Heat Exchangers. Learn about TEMA Class R, C, and B standards, the latest 10th edition updates, and how TEMA compares to ASME for mechanical design.

Master the engineering of vibration absorbers. Learn about Tuned Mass Dampers (TMD), design for resonant frequency, and how to specify absorbers for industrial assets.

Master the complexities of Steam Piping Design with our 2026 engineering guide. Covers ASME B31.1 standards, pipe sizing, condensate management, and safety.

Explore the essential Parts of a Heat Exchanger in this 2026 engineering guide. Covers tube sheets, baffles, shells, and technical solutions for optimal performance.

Master the fundamentals of Process Piping in 2026. Learn about ASME B31.3 codes, materials, and key differences between process piping, power piping, and plumbing.