What is a Condensate? Comprehensive Guide to Properties and Applications of Condensate

You are standing on the factory floor, and the steam traps are screaming. Every pound of steam that escapes as “waste” is direct profit evaporating into the atmosphere. Why is your plant treating high-purity, pre-heated water as a byproduct instead of a primary asset? Understanding the Properties and Applications of Condensate is the difference between a high-efficiency facility and a maintenance nightmare plagued by water hammer and rising fuel costs.
Key Takeaways
- Thermodynamic Value: Condensate contains roughly 25% of the heat energy of the original steam.
- Operational Safety: Managing condensate properties prevents catastrophic water hammer and carbonic acid corrosion.
- Sustainability: Efficient recovery can reduce boiler fuel consumption and water treatment costs by up to 20%.
What is Condensate?
Condensate is the liquid formed when steam transitions from a gaseous to a liquid state after releasing its latent heat. In industrial engineering, it is high-purity distilled water that retains significant sensible heat, making the Properties and Applications of Condensate vital for boiler feedwater and process heating efficiency.
“In over two decades of field audits, I have found that condensate is the most undervalued asset in the utility room. It is not just ‘hot water’; it is the most expensive water in your plant because you have already paid for its purification and thermal energy.”
– Atul Singla, Founder of Epcland
Table of Contents
Complete Course on
Piping Engineering
Check Now
Key Features
- 125+ Hours Content
- 500+ Recorded Lectures
- 20+ Years Exp.
- Lifetime Access
Coverage
- Codes & Standards
- Layouts & Design
- Material Eng.
- Stress Analysis
Engineering Knowledge Check
Test your mastery of the Properties and Applications of Condensate.
1. Approximately what percentage of the total energy in steam is typically retained by condensate as sensible heat?
1. What is a Condensate? Engineering Definition and Formation
In the context of industrial thermodynamics, condensate is the liquid phase that results when steam (water vapor) releases its latent heat of vaporization. This phase change occurs when steam comes into contact with a cooler surface or is used as a medium for heat transfer in equipment such as shell-and-tube heat exchangers, jacketed vessels, or radiators.
Understanding the Properties and Applications of Condensate starts with the formation process. As steam travels through a distribution system, it naturally loses energy to the environment due to radiation and convection. Once the steam gives up its latent heat (the energy required to maintain its gaseous state), it collapses back into a liquid. This liquid is distilled water of the highest purity, often at or near the saturation temperature corresponding to the system pressure.
From a chemical engineering standpoint, condensate is characterized by its lack of minerals and hardness, which were left behind in the boiler during the evaporation process. However, it is not “passive.” Once formed, condensate is highly aggressive; its high purity makes it a “hungry” solvent, often absorbing atmospheric gases like oxygen and carbon dioxide, which can lead to rapid degradation of carbon steel piping.
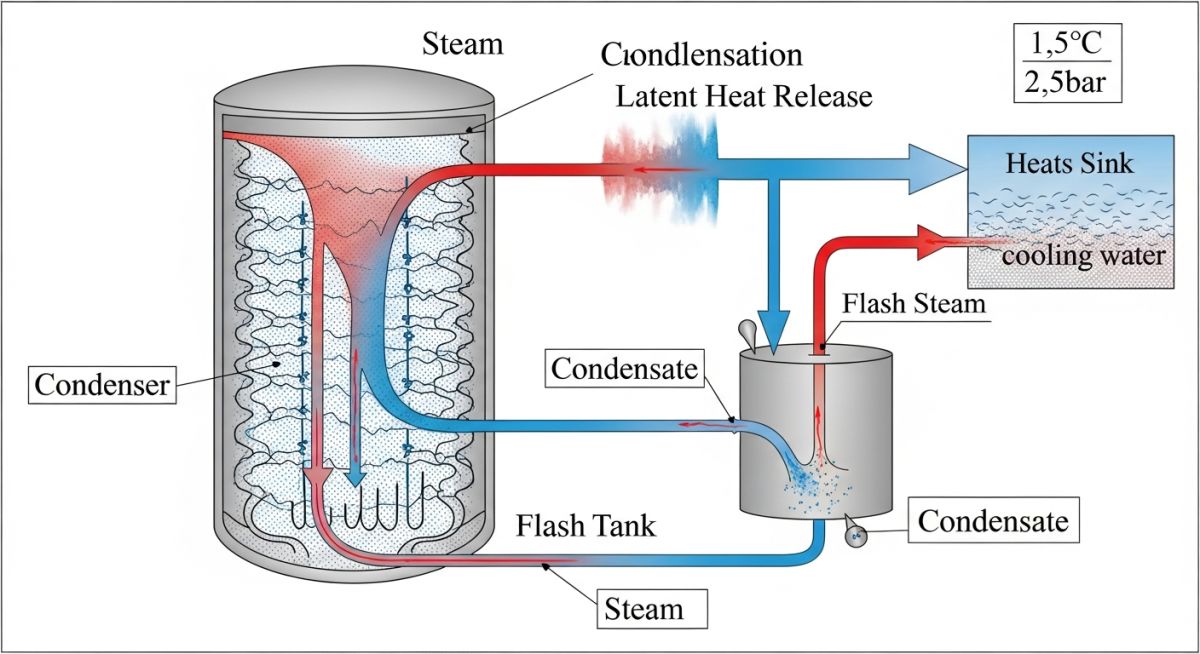
Figure 1: Thermodynamic phase transition and energy distribution in industrial condensate systems.
2. Physical and Thermodynamic Properties of Condensate
The Properties and Applications of Condensate are governed by the laws of thermodynamics, specifically the Steam Tables (IAPWS-IF97). To manage a steam system effectively in 2026, engineers must focus on three core physical properties:
-
01
Specific Enthalpy (hf): Condensate retains the “Sensible Heat” portion of the total heat of steam. For example, at 10 barg (145 psi), condensate at saturation temperature holds approximately 781 kJ/kg of energy. This represents about 25% of the energy initially invested in generating the steam.
-
02
Specific Volume (vf): The volume of condensate is significantly lower than steam. At 10 barg, the specific volume of steam is 0.163 m3/kg, while condensate is only 0.0011 m3/kg. This massive volume reduction (roughly 150:1) creates a vacuum effect in closed systems, which must be managed to prevent equipment collapse.
-
03
Flash Steam Potential: When high-pressure condensate is released to a lower pressure (such as a condensate return tank), it contains more energy than it can hold at the lower pressure. This excess energy causes a portion of the water to re-evaporate, a phenomenon known as “Flash Steam.”
In addition to thermodynamics, the chemical Properties and Applications of Condensate include its low conductivity and high potential for oxygen absorption. In 2026, real-time conductivity monitoring of condensate return lines is the industry standard for detecting process leaks (e.g., product contamination in a heat exchanger).
3. Industrial Applications of Condensate in Modern Power Plants
In the engineering landscape of 2026, the Properties and Applications of Condensate have shifted from simple disposal to complex resource recovery. High-pressure steam systems generate condensate that serves as a high-value thermal carrier.
Primary applications include Boiler Feedwater Pre-heating, where returned condensate (typically at 80°C to 95°C) reduces the fuel required to reach boiling point. In large-scale refineries, condensate is used for Process Heating via secondary heat exchangers, capturing remaining sensible heat for low-grade thermal requirements like tank farm heating or space warming.
4. Economic Benefits of Condensate Recovery Systems
The financial impact of optimizing the Properties and Applications of Condensate is measurable through reduced fuel consumption, lower water makeup costs, and minimized chemical treatment.
| Parameter | With Recovery (2026 Std) | Without Recovery (Waste) |
|---|---|---|
| Feedwater Temperature | 90°C – 105°C | 15°C – 25°C |
| Fuel Savings (%) | 12% – 20% Reduction | Baseline |
| Water Purity (TDS) | < 50 ppm | 250 – 500 ppm (Raw) |
| Blowdown Frequency | Minimal (Once/Shift) | Frequent (Continuous) |
5. Properties and Applications of Condensate: ASME & API Compliance
Professional management of condensate is dictated by several international standards. ASME PTC 12.2 provides the framework for steam surface condensers, while API RP 571 identifies the specific damage mechanisms, such as “Condensate Corrosion,” which occurs when non-condensable gases like CO2 dissolve into the liquid phase.
Engineers must also refer to ISO 15649 for piping systems in petroleum and natural gas industries to ensure that condensate return lines are sized for two-phase flow (water + flash steam) to avoid the catastrophic impacts of water hammer.
Flash Steam Recovery Calculator
Estimate the percentage of flash steam generated when high-pressure condensate is discharged. Understanding these Properties and Applications of Condensate helps in sizing recovery vessels.
Example: Sat. Liquid at 8 barg is ~763 kJ/kg
Example: Sat. Liquid at 0 barg is ~419 kJ/kg
Example: Latent heat at 0 barg is ~2257 kJ/kg
Based on these thermodynamic properties, 15.24% of your condensate will re-evaporate as flash steam.
Formula Used: Flash Steam % = ((hf1 – hf2) / hfg2) x 100. Data verified for 2026 industrial efficiency standards.
Don’t miss this video related to Condensate

Summary: Master Piping Engineering with our complete 125+ hour Certification Course: ……
Properties and Applications of Condensate Failure Case Study

The Scenario
In early 2026, a mid-sized chemical processing plant reported frequent pinhole leaks in their 4-inch carbon steel condensate return lines. Despite the condensate being “pure” distilled water, the piping was experiencing wall thinning rates of 1.5mm per year, far exceeding the design life.
Initial inspections suggested high-velocity erosion, but the flow rates were well within standard engineering limits. The plant was losing thousands in Properties and Applications of Condensate value due to lost treated water and thermal energy.
The Failure Root Cause
- Carbonic Acid Attack: CO2 from the boiler carryover was dissolving into the subcooled condensate, dropping pH levels to 5.5.
- Oxygen Pitting: Inefficient deaeration allowed dissolved oxygen to cause localized, deep pitting.
- Improper Pitch: Sections of the return line lacked the required 1:100 slope, leading to stagnant condensate pools.
The 2026 Engineering Resolution
The facility implemented a three-stage remediation plan: First, they installed a vacuum deaerator to strip non-condensable gases. Second, they switched to neutralizing amines for chemical pH control. Third, they upgraded the piping to 304L stainless steel in high-turbulence zones.
Expert Insights: Lessons from 20 years in the field
-
●
Prioritize Conductivity: In 2026, automation is key. Install online conductivity meters on all condensate return headers. A single heat exchanger leak can contaminate your entire boiler feed system in minutes.
-
●
The 1:100 Rule: Never underestimate the gravity. Ensure all condensate lines have a minimum downward pitch of 1:100 (10mm per meter) to prevent water hammer and stagnant zones.
-
●
Flash Steam is not “Smoke”: If you see white clouds above your condensate tank, that is lost money. In modern Properties and Applications of Condensate management, that flash steam should be captured for low-pressure heating.
-
●
Trap Testing: Implement an ultrasonic steam trap testing program twice a year. A failed-open trap is the primary cause of pressurized return line backpressure.
Frequently Asked Questions
What are the primary chemical properties and applications of condensate? ▼
Is condensate acidic or alkaline? ▼
Why does condensate cause water hammer? ▼
Why is my condensate return tank “steaming”? ▼
Does ASME require specific materials for condensate piping? ▼
How much fuel can I save by recovering 100% of my condensate? ▼
References & Standards
📚 Recommended Resources: Condensate
Read these Guides








