Oxidation State of Nickel: Chemistry, Calculation, and Engineering Applications (2026 Guide)

The Oxidation State of Nickel is a fundamental concept in inorganic chemistry and metallurgy that dictates how this versatile transition metal interacts with its environment. Understanding these valence changes is critical for designing corrosion-resistant superalloys, high-efficiency batteries, and industrial catalysts. This guide explores the thermodynamic stability, calculation methods, and engineering implications of nickel’s variable oxidation numbers in 2026.
What is the Oxidation State of Nickel?
The Oxidation State of Nickel refers to the hypothetical charge an atom of nickel would have if all bonds to atoms of different elements were 100% ionic. While nickel exhibits oxidation states ranging from -1 to +4, the +2 state (Ni(II)) is the most stable and common in nature, forming a wide array of green-colored coordination complexes and simple oxides like NiO.
Table of Contents
- 1. Fundamentals: What Defines the Oxidation State of Nickel?
- 2. The Chemistry Behind Nickel Oxidation
- 3. Common Nickel Oxidation States (+2, +3, +4)
- 4. Calculating the Nickel Oxidation Number
- 5. The Nickel Oxidation Mechanism
- 6. How Oxidation State Affects Reactivity
- 7. Critical Parameters Influencing Oxidation State Transitions
- 8. Engineering Applications of Nickel Oxidation
- 9. Case Study: Superalloy Failure Analysis
- 10. Frequently Asked Questions
Test Your Knowledge: Nickel Chemistry
Loading…
Quiz Complete!
Complete Course on
Piping Engineering
Check Now
Key Features
- 125+ Hours Content
- 500+ Recorded Lectures
- 20+ Years Exp.
- Lifetime Access
Coverage
- Codes & Standards
- Layouts & Design
- Material Eng.
- Stress Analysis
1. Fundamentals: What Defines the Oxidation State of Nickel?
In the context of inorganic chemistry and materials engineering, the Oxidation State of Nickel is a measure of the degree of oxidation of a nickel atom in a chemical compound. It represents the number of electrons that the nickel atom has effectively lost (positive state) or gained (negative state) compared to its neutral elemental form.
For metallurgists and corrosion engineers, monitoring the Oxidation State of Nickel is crucial because it dictates the metal’s passivity. In superalloys (like Inconel or Hastelloy), nickel exists primarily in the metallic state (0) but relies on surface passivation—often forming a thin layer of Ni(II) Oxide (NiO)—to resist degradation in harsh environments.
2. The Chemistry Behind Nickel Oxidation
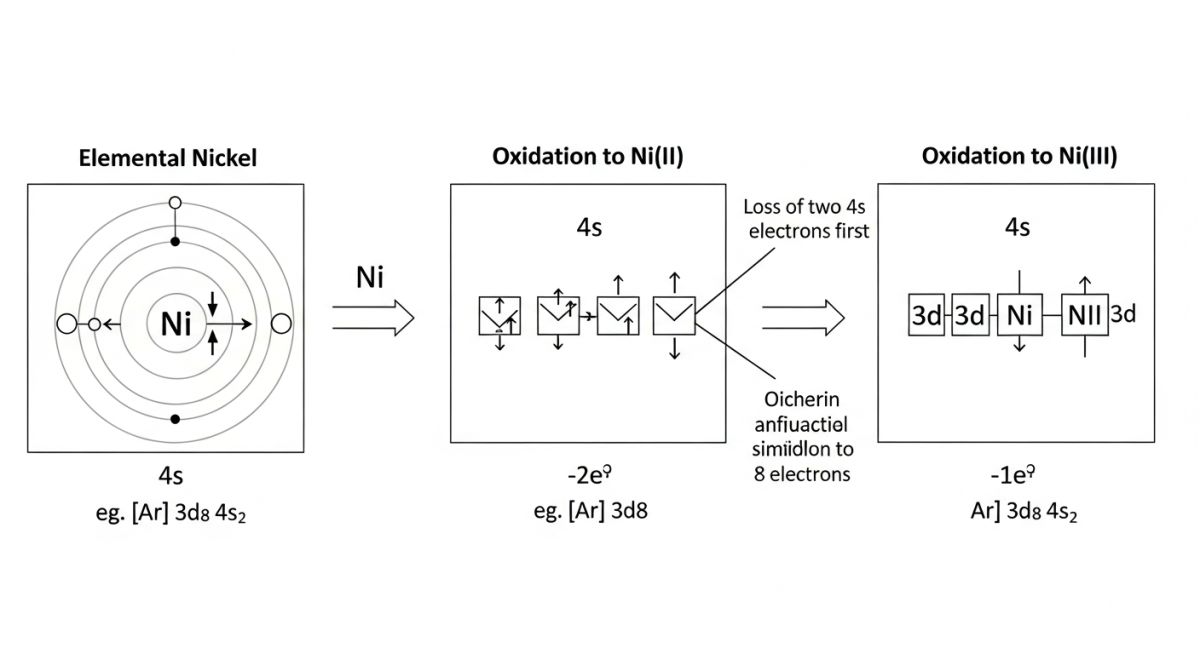
To understand the Oxidation State of Nickel, we must look at its electron configuration. Neutral nickel (Atomic Number 28) has a configuration of [Ar] 3d8 4s2.
- Ni(0): The neutral metal found in alloys. All 28 electrons are present.
- Ni(II): The most common oxidized form. Nickel loses the two 4s electrons first, resulting in a [Ar] 3d8 configuration. This d8 arrangement is highly stable in octahedral coordination fields.
- Ni(III): Formed by losing one additional electron from the 3d orbital ([Ar] 3d7). This state is a strong oxidizing agent and is typically unstable unless stabilized in specific crystal lattices or battery cathodes.
3. Common Nickel Oxidation States (+2, +3, +4) and Their Stability
The Dominant +2 State (NiO and Salts)
The +2 Oxidation State of Nickel is the most chemically stable. Compounds like Nickel Oxide (NiO), Nickel Chloride (NiCl2), and Nickel Sulfate (NiSO4) all feature nickel in this state. In aqueous solutions, the hexaaquanickel(II) ion [Ni(H2O)6]2+ gives these solutions a characteristic emerald green color.
Higher Oxidation States of Nickel (+3, +4)
Higher Nickel Oxidation States like +3 and +4 are rare in nature but vital for energy storage. In Nickel-Metal Hydride (NiMH) and Nickel-Cadmium (NiCd) batteries, the charging cycle forces nickel from +2 to +3 (as NiOOH). These states are powerful oxidizers and revert to +2 during battery discharge.
4. Calculating the Nickel Oxidation Number in Coordination Complexes
Determining the correct Nickel Oxidation Number requires summing the charges of all ligands and equating them to the total charge of the molecule. The formula is:
Equation:
XNi + ∑(ChargeLigands) = Total ChargeComplex
Example 1: Nickel Chloride (NiCl2)
- Chlorine (Cl) has a standard charge of -1.
- The molecule is neutral (Total Charge = 0).
- Calculation: X + 2(-1) = 0 → X = +2.
- Result: The Oxidation State of Nickel is +2.
Example 2: Tetracyanonickelate(II) Ion [Ni(CN)4]2-
- Cyanide (CN) is a ligand with a -1 charge.
- The complex ion has a total charge of -2.
- Calculation: X + 4(-1) = -2 → X – 4 = -2 → X = +2.
- Result: Despite the complex structure, the Nickel Oxidation Number remains +2.
5. The Nickel Oxidation Mechanism: Kinetics and Thermodynamics
The transition from metallic nickel to a specific Oxidation State of Nickel follows governed kinetic laws, particularly at high temperatures. The Nickel Oxidation Mechanism is primarily controlled by the diffusion of Nickel ions (Ni2+) outward through the oxide lattice and oxygen ions (O2-) inward.
The Parabolic Rate Law
For temperatures above 400°C, the growth of the NiO layer typically follows the parabolic rate law, indicating that the reaction is diffusion-limited. The thickness of the oxide layer (x) over time (t) is described by:
Where kp is the parabolic rate constant (dependent on the Oxidation State of Nickel stability at that temperature) and C is the integration constant.
6. How the Oxidation State of Nickel Affects Compound Reactivity
The chemical behavior of nickel compounds is strictly dictated by the Oxidation State of Nickel.
- Ni(II) Reactivity: Generally acts as a weak reducing agent. It is stable in acids but precipitates as nickel hydroxide in alkaline solutions.
- Ni(III)/Ni(IV) Reactivity: These higher Nickel Oxidation States are strong oxidizing agents. They react aggressively with water or acids to revert to the more stable +2 state, releasing oxygen gas in the process.
Comparison of Nickel Compounds by Oxidation State
| Compound | Oxidation State | Stability (25°C) | Engineering Application |
|---|---|---|---|
| Nickel Oxide (NiO) | +2 | High | Ceramics, Catalysts, Passivation Layers |
| Nickel Hydroxide Ni(OH)2 | +2 | Moderate | Battery Electrodes (Discharged State) |
| Nickel Oxyhydroxide (NiOOH) | +3 | Low (Metastable) | Ni-MH/Ni-Cd Batteries (Charged State) |
| Potassium Hexafluoronickelate | +4 | Very Low | Specialized Oxidizing Agents |
7. Critical Parameters Influencing Nickel Oxidation State Transitions
Controlling the Oxidation State of Nickel is essential for industrial processes. The transition from metallic Ni to Ni(II) or Ni(III) depends on several environmental variables defined by API and NACE standards.
Oxygen Availability and Partial Pressure
The thermodynamic stability of the Oxidation State of Nickel is directly linked to oxygen partial pressure (pO2). At low pressures, Ni remains metallic. As pO2 increases, the equilibrium shifts to form NiO. In high-pressure industrial boilers, excess oxygen can drive localized pitting if the passive film is breached.
Temperature Effects on Nickel Oxidation Rates
Temperature acts as an exponential accelerator. Below 300°C, the Oxidation State of Nickel stabilizes as a thin, invisible passive film. Above 600°C (common in gas turbines), the oxidation rate increases rapidly, potentially leading to “breakaway oxidation” if the protective NiO scale cracks.
pH Level: Acidic vs. Alkaline Stability
The Pourbaix diagram for nickel shows that the Oxidation State of Nickel (+2) is soluble in acidic solutions (pH < 6.5), existing as Ni2+ ions. In alkaline environments (pH > 8.5), it precipitates as stable solid Ni(OH)2 or NiO, which is crucial for corrosion protection in seawater applications.
Presence of Impurities (Sulfur/Phosphorus)
Impurities like Sulfur can disrupt the stable Nickel Oxidation State. Sulfur reacts with nickel to form Nickel Sulfide (Ni3S2), a compound with a low melting point (eutectic forms at 635°C). This liquid phase destroys the protective oxide layer, a phenomenon known as “hot corrosion.”
Presence of Reducing Agents
In environments containing Hydrogen or Carbon Monoxide, the Oxidation State of Nickel can be reduced from +2 back to 0. This is the fundamental principle behind the “Bright Annealing” of stainless steels, where a reducing atmosphere prevents the formation of visible oxide scales.
Nickel Oxide (NiO) Scale Growth Calculator
Estimate the thickness of the oxide scale formed on Nickel surfaces at high temperatures using the Parabolic Rate Law ($x = \sqrt{k_p \cdot t}$). This calculation is critical for predicting the lifespan of superalloy components in oxidation environments.
Typical values: 0.01 (600°C) to 1.5 (1000°C) depending on alloy.
Estimated Oxide Thickness
Awaiting Input
EPCLand YouTube Channel
2,500+ Videos • Daily Updates
11. Advanced Alloys: Controlling the Oxidation State of Nickel in Industry
While the Oxidation State of Nickel (+2) forms a passive film (NiO), this layer is often insufficient for extreme industrial environments. Pure Nickel (Ni-200) suffers from rapid scaling above 600°C. To combat this, engineers utilize superalloys that modify the oxidation mechanism, suppressing the formation of unstable nickel oxides in favor of more robust protective layers like Chromia (Cr2O3) or Alumina (Al2O3).
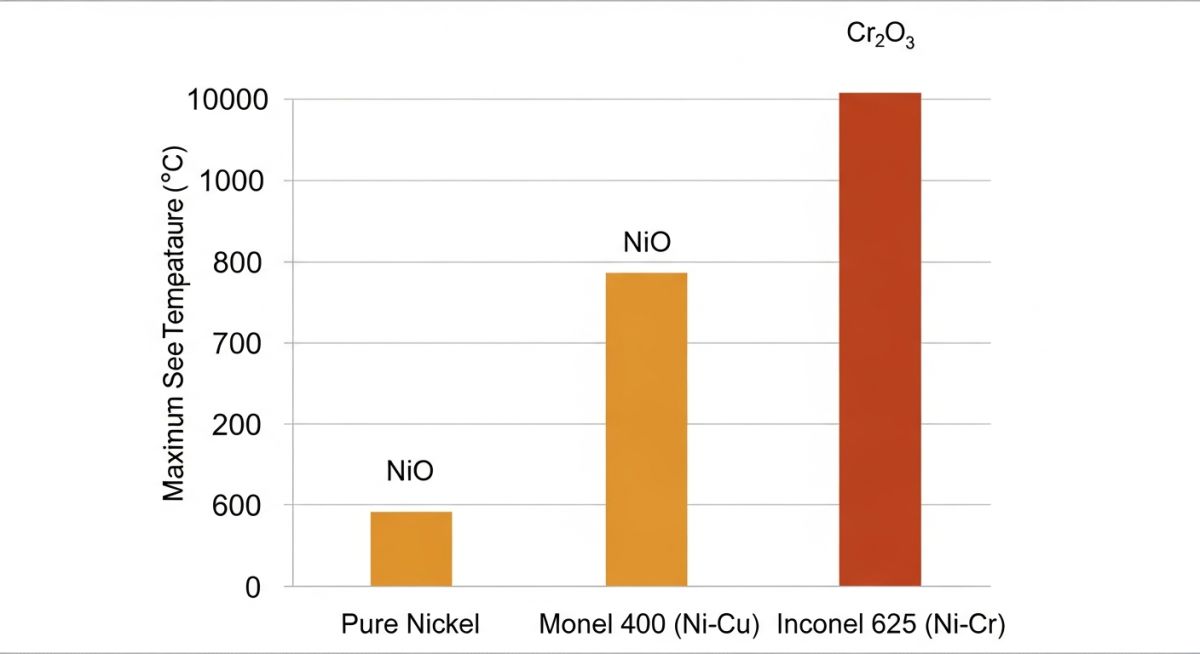
The Chromia Effect
In alloys like Inconel, Chromium oxidizes preferentially to Nickel. Even though Nickel constitutes the bulk of the matrix, the surface forms a Cr2O3 layer. This effectively "locks" the Nickel in its metallic (0) state beneath the surface, preventing the uncontrolled progression to the +2 Oxidation State of Nickel that leads to failure.
Material Selection Guide (2026 Standards)
| Alloy Family | Primary Composition | Oxidation Behavior | Max Service Temp |
|---|---|---|---|
| Ni-200 (Pure Nickel) | 99% Ni | Relies solely on NiO formation. Good in reducing environments but scales rapidly in oxidizing heat. | 600°C |
| Monel 400 | 67% Ni, 23% Cu | Copper destabilizes the Oxidation State of Nickel at high temps. Excellent in sea water/hydrofluoric acid. | 500°C |
| Inconel 625 | 58% Ni, 21% Cr, 9% Mo | Chromium forms a superior barrier. Nickel acts only as the solid solution matrix, not the primary reactant. | 980°C |
| Incoloy 800HT | 32% Ni, 21% Cr, 46% Fe | Balanced specifically for resistance to oxidation and carburization in petrochemical furnaces. | 1100°C |
Selection Rule of Thumb: For environments where the Oxidation State of Nickel is liable to fluctuate rapidly (e.g., cyclic heating/cooling), High-Chromium superalloys are mandatory to prevent spallation. For reducing acids (HCl, HF), Nickel-Copper (Monel) or Pure Nickel is preferred as they resist the formation of soluble Ni(II) salts.
8. Engineering Applications of Various Nickel Oxidation States
Leveraging the specific chemical properties of the Oxidation State of Nickel allows engineers to design systems ranging from massive industrial reactors to microscopic electronic components.
Catalysis (Ni-0/Ni-II)
Raney Nickel (Ni-0) is used for hydrogenation of vegetable oils. Ni(II) complexes serve as precursors for cross-coupling reaction catalysts.
Electroplating
Watts baths use Ni(II) sulfate and chloride to deposit a layer of metallic nickel (reduced from +2 to 0) for corrosion resistance.
Batteries (Ni-III)
The reversible transition between Ni(II) hydroxide and Ni(III) oxyhydroxide drives the energy storage in Ni-MH and Ni-Cd cells.
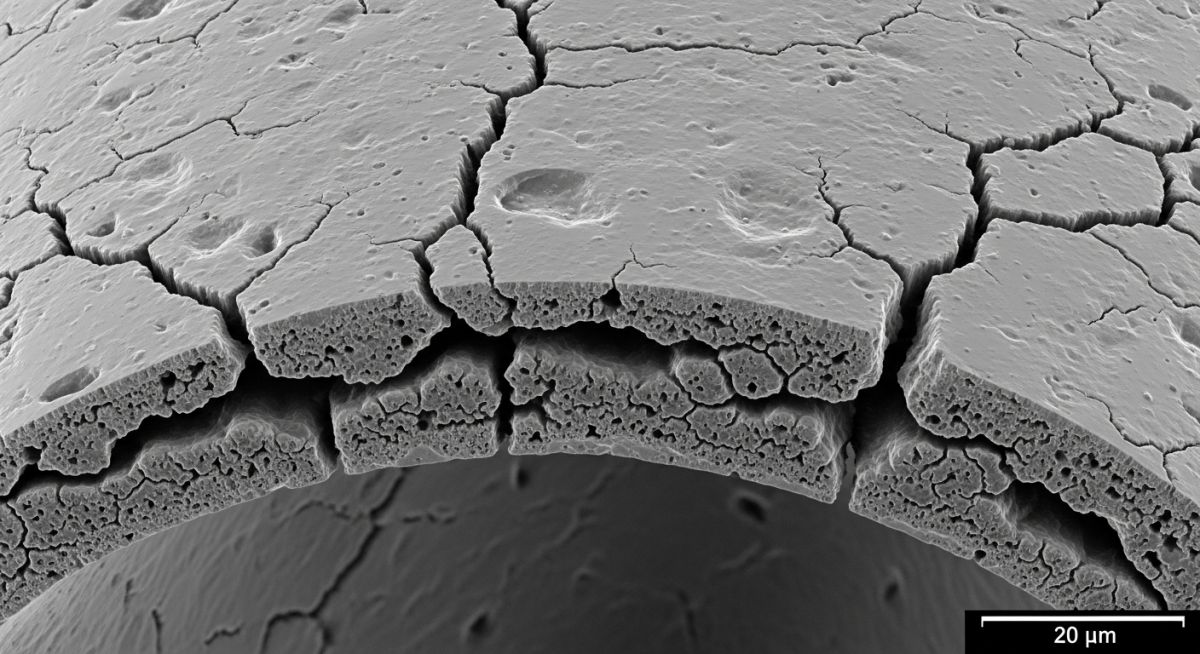
9. Case Study: Oxidation State of Nickel in Superalloy Failure Analysis
Component
Gas Turbine Blade (Stage 1)
Material
Ni-Based Superalloy (70% Ni)
Temp
1050°C Peak
Failure Mode
Accelerated Oxidation
The Incident
During a routine 24,000-hour inspection in early 2026, severe spallation was observed on the leading edge of the turbine blades. The material loss threatened the aerodynamic efficiency and structural integrity of the rotor.
Root Cause Analysis
Metallographic analysis revealed that the protective mechanism relied on the stable Oxidation State of Nickel (+2) forming a continuous NiO scale. However, due to temperature excursions above 1000°C, the growth rate of the Ni(II) oxide layer exceeded the parabolic limit.
The thick NiO layer developed high internal compressive stresses, leading to cracking. Once the scale cracked, oxygen penetrated directly to the substrate, causing rapid consumption of the nickel matrix and forming non-protective, porous oxides.
Engineering Fix
The solution involved modifying the surface chemistry. A platinum-aluminide coating was applied. This coating preferentially forms Alumina (Al2O3) instead of Nickel Oxide. Alumina remains stable and slow-growing at temperatures where the Oxidation State of Nickel leads to unstable oxide growth, effectively shielding the nickel substrate.
Key Lesson
Reliance solely on the passivation of the +2 Oxidation State of Nickel is insufficient for applications exceeding 900°C. Alloying elements (Cr, Al) are required to suppress rapid nickel oxidation.
10. Frequently Asked Questions about Nickel Oxidation
What is the most stable Oxidation State of Nickel in aqueous solutions?
Can Nickel exist in negative oxidation states?
How does the Nickel Oxidation Number impact magnetic properties?
Why is the +3 state important if it is unstable?
Conclusion: Mastering Nickel Chemistry in 2026
From the protective oxide scales on jet engine turbine blades to the cathodes of rechargeable batteries, the chemistry of nickel is ubiquitous in modern engineering. Success in these fields depends on the precise control of thermodynamics and kinetics.
By understanding the factors that influence the Oxidation State of Nickel—including temperature, pH, and ligand environments—engineers can predict failure modes, optimize catalytic processes, and develop the next generation of energy storage solutions.
📚 Recommended Resources: Oxidation
Read these Guides








